麻省理工學院(MIT)開發出一種可從空氣中去除二氧化碳的新方法,有機會成為對抗氣候變遷的利器。
要從其他氣體流中去除二氧化碳,大多數方法都需要相當高的濃度,如化石燃料發電廠排煙管的濃度,而MIT的新技術可以應用在幾乎任何濃度的二氧化碳,甚至可低至400ppm。研究人員說,目前已經有一些可應用於低濃度的新技術,但是MIT的新方法耗能較低、成本也較低。
MIT博士後研究員沃斯基安(Sahag Voskian)在博士班期間開發出這個系統。他和化工學系教授哈頓(T. Alan Hatton)將本研究發表於《能源與環境科學》(Energy and Environmental Science) 期刊。
這個新技術的基本流程是讓空氣通過一組帶電的電化學板。整個設備是個可從空氣或其他氣體流中吸收二氧化碳的大型特殊電池,充電時空氣流過其電極,放電時釋放出氣體。
運作時,該設備就是不斷重複充電跟放電,充電時將新鮮空氣或原料氣體吹入系統,放電過程中吹出純濃縮二氧化碳。
隨著電池充電,電極堆中每一個電極的表面發生電化學反應。這些電極上塗著奈米碳管複合而成的聚蒽醌(polyanthraquinone)。這些電極對二氧化碳具有天然的親和力,即使在濃度很低的情況下也很容易與空氣或進料氣體中的分子發生反應。
電池放電時會發生逆反應。在此過程中,該設備可以提供整個系統所需的部分電源,並且可以噴射出純的二氧化碳。整個系統在室溫和正常氣壓下運行。
沃斯基安解釋:「與大多數其他碳捕獲或碳吸收技術相比,這個技術的最大優勢是吸附劑對二氧化碳的親和力具有二元性質。」
也就是說,電極材料的性質「要不親和力很高,要不沒有親和力」,取決於電池的充電或放電狀態。其他碳捕獲技術的反應則需要中間化學處理步驟或熱量、壓力差等能量輸入。
「這種二元親和力可以捕獲任何濃度的二氧化碳,包括400ppm,並可釋放到任何載流中,包括100%二氧化碳。」
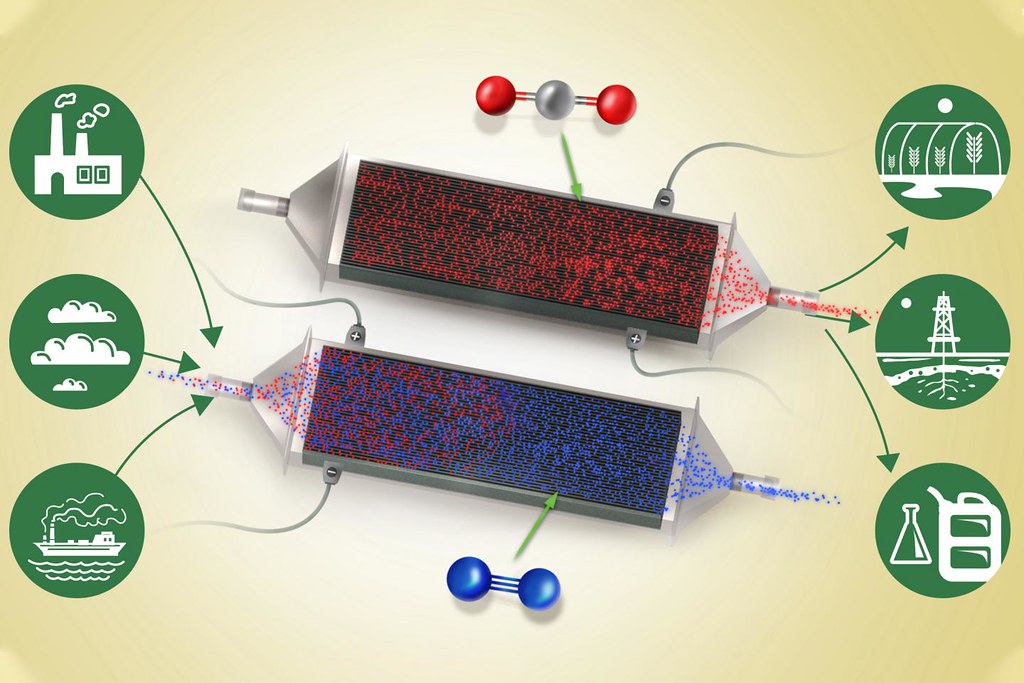
部分軟性飲料裝瓶廠會燃燒化石燃料以產生二氧化碳,用於製作氣泡飲料。同樣地,有些農民會燃燒天然氣產生二氧化碳,飼育溫室裡的植物。沃斯基安說,新系統可以讓這些應用不再需要化石燃料,也可以將溫室氣體從空氣中清除。
或者也可以將純二氧化碳氣流壓縮,注入地下以進行長期處理,甚至透過一系列化學和電化學過程製成燃料。
沃斯基安說,這個系統捕獲和釋放二氧化碳的過程都是革命性的進展,「所有過程都在室溫發生,不需要熱、壓力或化學輸入,只需這些兩個表面都能作用的薄片,可以堆疊在盒子中並接上電源。」
在工廠中,像是會不斷產生廢氣的發電廠,可以並排設置兩組這樣的電化學電池堆同時交替運作,將煙氣導向第一組進行碳捕集,第一組進入放電狀態時就導到第二組,如此系統可以一直同時捕獲和排出氣體。
在實驗室中,該團隊已經證明該系統可以承受至少7,000次充放電循環,在這段時間內效率損失30%。研究人員估計,他們可以輕鬆地將其提高到20,000至50,000個循環。
電極本身可以用標準化學處理方法製造。沃斯基安說,雖然目前製造工作仍是在實驗室環境中完成,但可以調整成用類似報紙印刷機的卷對卷製造工藝大量生產。他說:「我們已經開發出非常具有成本效益的技術,」他估計,這種材料的生產成本大約為每平方公尺的電極數十美元。
與其他現有的碳捕獲技術相比,該系統能源效率很高,每捕獲一噸二氧化碳使用約10億焦耳(1 GJ)的能量,而且始終如一。沃斯基安說,其他現有方法的能耗依進氣二氧化碳濃度不同,每噸介於10億到100億焦耳(10 GJ)之間。
研究人員成立了一家名叫Verdox的公司,以將該技術商業化,並希望在未來幾年內打造出先導性規模的工廠。該系統非常容易擴展。沃斯基安說:「如果需要更大的容量,只需要製造更多的電極即可。」
A new way of removing the greenhouse gas carbon dioxide (CO2) from a stream of air could provide a valuable tool in the battle against climate change say the engineers at the Massachusetts Institute of Technology, MIT, who developed the new system.
It can work on the heat-trapping gas at virtually any concentration level, even down to the roughly 400 parts per million currently found in the atmosphere.
Most methods of removing CO2 from a stream of other gases require higher concentrations, such as those found in the flue emissions from fossil fuel-based power plants. A few variations have been developed that can work with the low concentrations found in air, but the new method is less energy-intensive and expensive, the researchers say.
The technique, based on passing air through a stack of charged electrochemical plates, is described in a new paper in the journal “Energy and Environmental Science,” by MIT postdoc Sahag Voskian, who developed the work during his PhD, and T. Alan Hatton, the Ralph Landau professor of chemical engineering.
The device is a large, specialized battery that absorbs carbon dioxide from the air, or other gas stream, passing over its electrodes as it is being charged up, and then releases the gas as it is being discharged.
In operation, the device would simply alternate between charging and discharging, with fresh air or feed gas being blown through the system during the charging cycle, and then the pure, concentrated carbon dioxide being blown out during the discharging.
As the battery charges, an electrochemical reaction takes place at the surface of each of a stack of electrodes. These are coated with a compound called polyanthraquinone, which is composited with carbon nanotubes. The electrodes have a natural affinity for carbon dioxide and readily react with its molecules in the airstream or feed gas, even when it is present at very low concentrations.
The reverse reaction takes place when the battery is discharged. During this process, the device can provide part of the power needed for the whole system, and it ejects a stream of pure carbon dioxide. The whole system operates at room temperature and normal air pressure.
“The greatest advantage of this technology over most other carbon capture or carbon-absorbing technologies is the binary nature of the adsorbent’s affinity to carbon dioxide,” explains Voskian.
In other words, the electrode material, by its nature, “has either a high affinity or no affinity whatsoever,” depending on the battery’s state of charging or discharging. Other reactions used for carbon capture require intermediate chemical processing steps or the input of energy such as heat or pressure differences.
“This binary affinity allows capture of carbon dioxide from any concentration, including 400 parts per million, and allows its release into any carrier stream, including 100 percent CO2,” Voskian says.
In some soft-drink bottling plants, fossil fuel is burned to generate the carbon dioxide needed to give the drinks their fizz. Similarly, some farmers burn natural gas to produce carbon dioxide to feed their plants in greenhouses. The new system could eliminate that need for fossil fuels in these applications, and in the process actually be taking the greenhouse gas right out of the air, says Voskian.
Alternatively, the pure CO2 stream could be compressed and injected underground for long-term disposal, or even made into fuel through a series of chemical and electrochemical processes.
The process this system uses for capturing and releasing carbon dioxide “is revolutionary,” Voskian says. “All of this is at ambient conditions – there’s no need for thermal, pressure, or chemical input. It’s just these very thin sheets, with both surfaces active, that can be stacked in a box and connected to a source of electricity.”
In a working plant, for example, in a power plant where exhaust gas is being produced continuously, two sets of such stacks of the electrochemical cells could be set up side by side to operate in parallel, with flue gas being directed first at one set for carbon capture, then diverted to the second set while the first set goes into its discharge cycle.
By alternating back and forth, the system could always be both capturing and discharging the gas.
In the lab, the team has proven the system can withstand at least 7,000 charging-discharging cycles, with a 30 percent loss in efficiency over that time. The researchers estimate that they can readily improve that to 20,000 to 50,000 cycles.
The electrodes themselves can be manufactured by standard chemical processing methods. While today this is done in a laboratory setting, it can be adapted so that ultimately they could be made in large quantities through a roll-to-roll manufacturing process similar to a newspaper printing press, Voskian says. “We have developed very cost-effective techniques,” he says, estimating that it could be produced for something like tens of dollars per square meter of electrode.
Compared to other existing carbon capture technologies, this system is quite energy efficient, using about one gigajoule of energy per ton of carbon dioxide captured, consistently. Other existing methods have energy consumption which varies between one to 10 gigajoules per ton, depending on the inlet carbon dioxide concentration, Voskian says.
The researchers have set up a company called Verdox to commercialize the process, and hope to develop a pilot-scale plant within the next few years. And the system is very easy to scale up. Voskian says, “If you want more capacity, you just need to make more electrodes.”
※ 全文及圖片詳見:ENS









